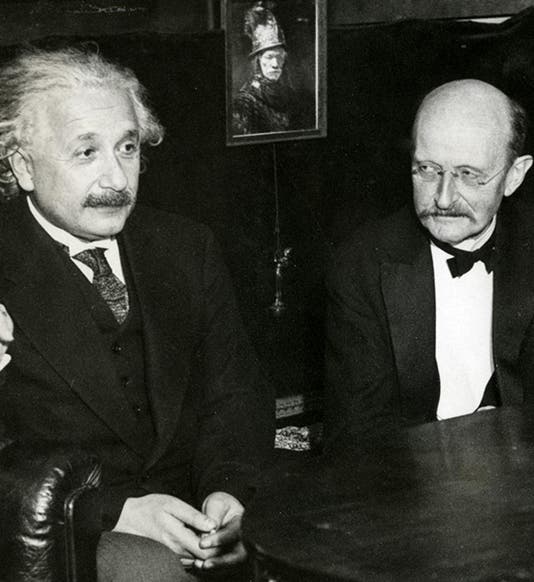Scientist of the Day - Max Planck
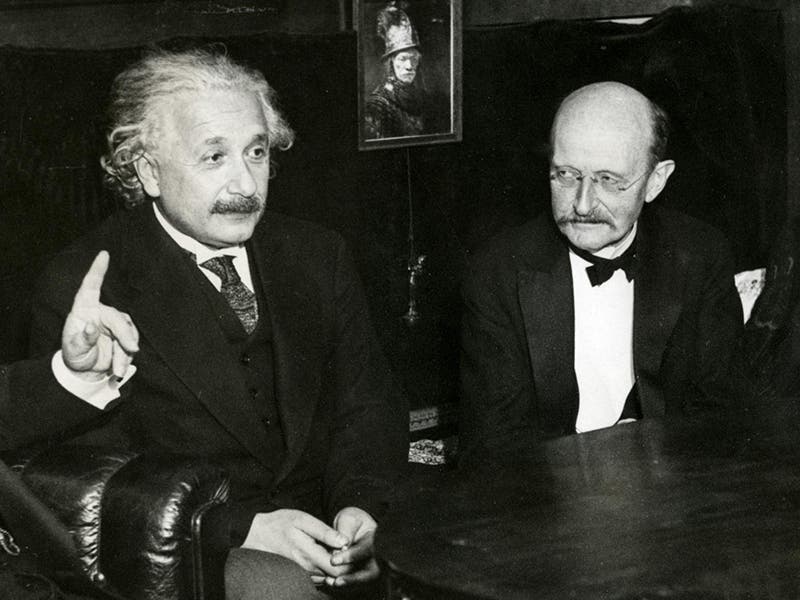


Max Planck, a German physicist, was born Apr. 23, 1858. In the late 1890s, Planck was trying to explain a paradox called the "ultraviolet catastrophe." Theory predicted that certain objects called "black bodies" should emit prohibitively prodigious amounts of ultraviolet radiation. In reality, this does not occur. Planck found he could explain the paradox by proposing that energy is not infinitely divisible, but comes in small minimal packets. Planck called such a packet a "quantum" of energy. Thus, in 1900, the quantum theory was born. When, five years later, Einstein successfully applied the concept of quanta to light, quantum mechanics was off and running, and it runs with us still (the first image above shows Einstein and Planck at a later date). It is interesting that, at the first Solvay conference for physicists in 1911 (a group photo is the second image above), when neither relativity theory nor quantum theory were well accepted, Planck was relegated to the background (second from left in the rear), and Einstein to the sidelines, while center stage was held by Marie Curie. Sixteen years later, at the fifth Solvay conference in 1927 (third image above), Einstein and Planck have been moved to the front row. Marie Curie has retained her distinguished position between the two, as indeed she should have. The only unfortunate side-effect of Planck’s quantum theory is a misappropriated expression that is in common use today. We often employ the term "quantum leap" to denote some sort of large-scale change, when in fact a quantum leap is the very smallest transition that can possibly occur in nature. Ah, the English language! Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City