Scientist of the Day - Paul Emile Lecoq de Boisbaudran
Linda Hall Library
Linda Hall Library
Paul Émile Lecoq de Boisbaudran, a French chemist, was born Apr. 18, 1838. De Boisbaudran was a pioneer in spectroscopic chemistry, by which new elements can be identified in compounds when unfamiliar lines show up in the compound's spectra. De Boisbaudran published the first French book on chemical spectroscopy, Spectres lumineux, in 1874. Putting his methods to good use, De Boisbaudran discovered four new elements in all, including samarium and europium. But his most famous discovery was gallium, which he detected spectroscopically in 1875 and isolated later that year.
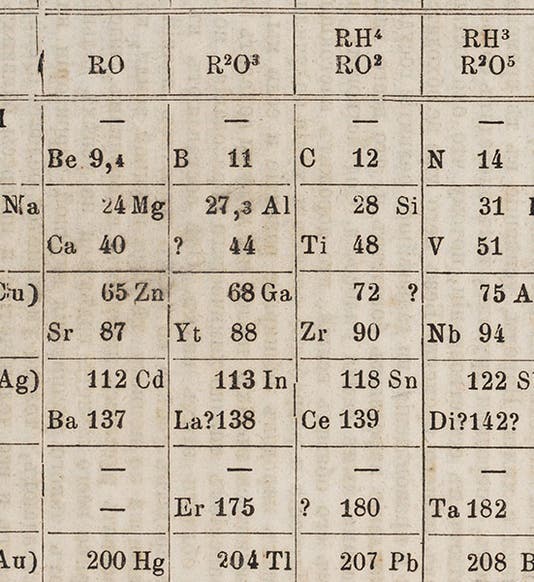
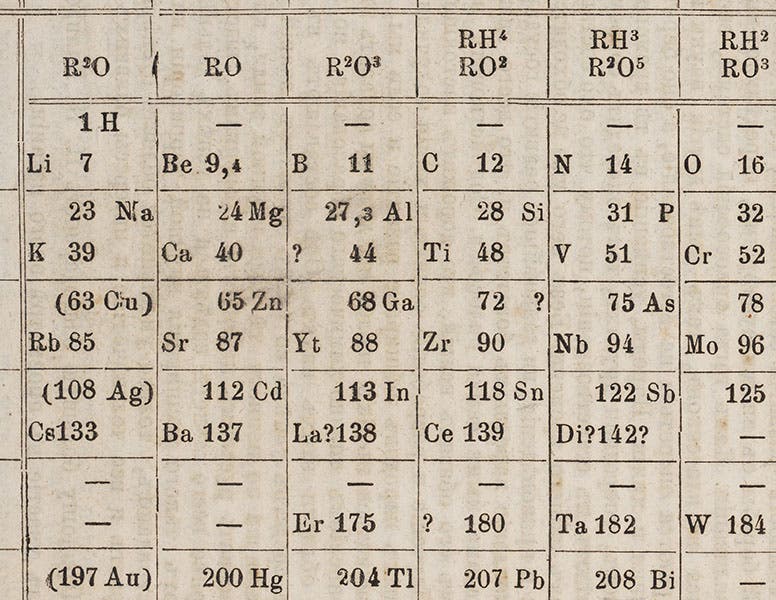
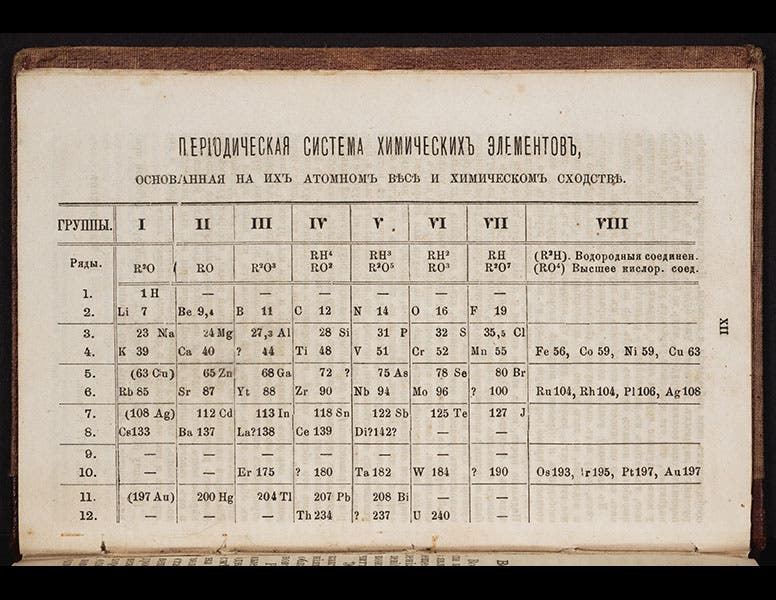
In 1869, Dmitrii Mendeleev had published his first periodic table. There were three notable "holes" in the table, places were elements would fit, and should fit, but which did not exist, at least according to contemporary chemical knowledge. Mendeleev, very sure of the soundness of his periodic arrangement, predicted that they do exist, and he even gave them names, based on the properties they should have by their place in his table. He called them "eka-boron," "eka-silicon," and "eka-aluminum", and in a way, he challenged chemists to find them.
De Boisbaudran was the first to do so. His new element of 1875, which had an atomic weight of about 69, was very close to Mendeleev's predicted atomic weight for "eka-aluminum", 68. De Boisbaudran claimed that he named it “gallium” after the Latin word for France, but some suspected that he really named it after himself, since "Le Coq" means rooster, and rooster in Latin is "Gallus". Chemical names need more double meanings like this.
Mendeleev later claimed to have discovered gallium himself, since his predicted properties, especially the density, turned out to be better than those that de Boisbaudran measured, which did not sit well with de Boisbaudran, or with lab chemists in general. Today, de Boisbaudran gets the credit, although you will never see a discussion of de Boisbaudran that does not include Mendeleev.
Gallium first shows up in a periodic table in the third edition of Mendeleev’s Principles of Chemistry (1877), which we just happen to have acquired last year for the Library (detail in first image; complete table, second image).
Gallium (fourth image) is an interesting metal, in that it melts at 86°F, lower than body temperature. So if you were to give someone a pair of glasses with gallium frames, the frames would soon begin to sag and droop around the nose and ears, the ocular version, one supposes, of a gallic shrug.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.