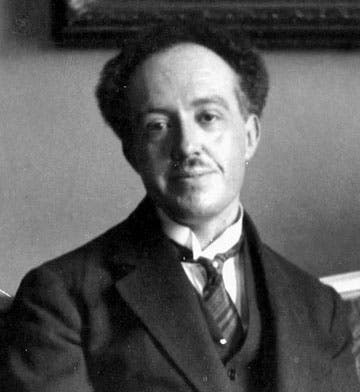Scientist of the Day - Louis de Broglie
Louis Victor, 7th Duc de Broglie, a French aristocrat and physicist, died Mar. 19, 1987, at the age of 94. De Broglie started out intending to study history and politics, but he turned to math and physics, perhaps influenced by his older brother Maurice (16 years older), who became the 6th Duc in 1906 (Louis would not inherit the title until 1960). Maurice investigated X-rays and X-ray diffraction in the years before World War I, and Louis seems to have taken an interest in the subject. He spent 6 years in the French army during the War, working on radio communications, which set back his budding career in physics just a bit. But he made up for lost time in a hurry, as we shall see.
If you studied physics in 1920, you knew that Niels Bohr had discovered the quantum nature of the atom. Bohr found that the electrons in the hydrogen atom were confined to discrete orbits with prescribed amounts of energy. To move from a higher orbit to a lower one, an electron emitted a photon, a quantum of light, and shifted instantaneously to the lower orbit. Similarly, when an electron absorbed a photon of a certain wavelength (which Bohr could predict), it gained energy and jumped (a quantum leap) to a higher orbit.
De Broglie was also aware that light behaves like both a particle and a wave, as Albert Einstein had shown in 1905. However, in 1920, only light exhibited particle-wave duality. Electrons and protons (the latter were discovered in 1919) were considered to be particles all the way down.
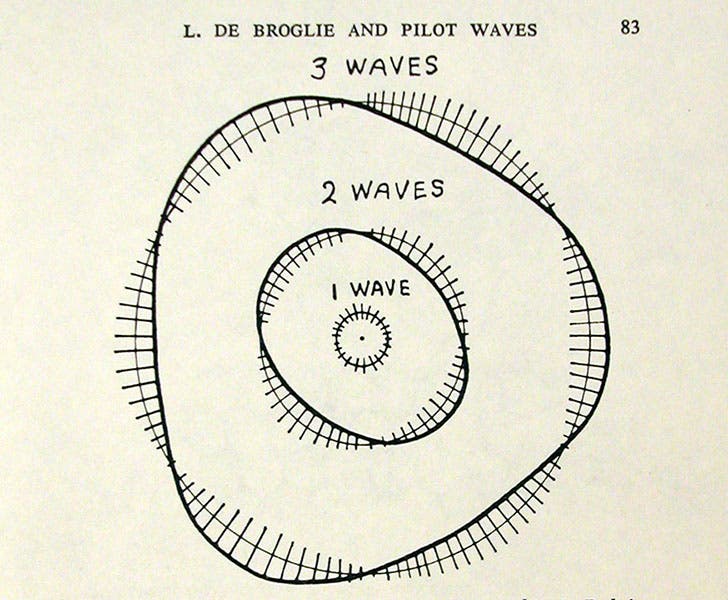
Electron waves fitted to quantum orbits in the Bohr atom, according to Louis de Broglie, drawing by George Gamow in his Thirty Years that Shook Physics, 1966 (author’s copy)
De Broglie, as he prepared for his doctoral degree, wondered whether other forms of matter might have associated waves; in particular, might an electron have an associated wave, just like a photon? He mused about this as he tried to understand why an electron can occupy one Bohr orbit or another, but could not be anywhere in between. What if an electron, he proposed, has a wave associated with it whose wavelength fits exactly into the first Bohr orbit. And what if the second orbit would accommodate two electron waves, and the third, three, and so forth? It would easily make sense of the discrete orbits of the Bohr atom (see diagram, second image).
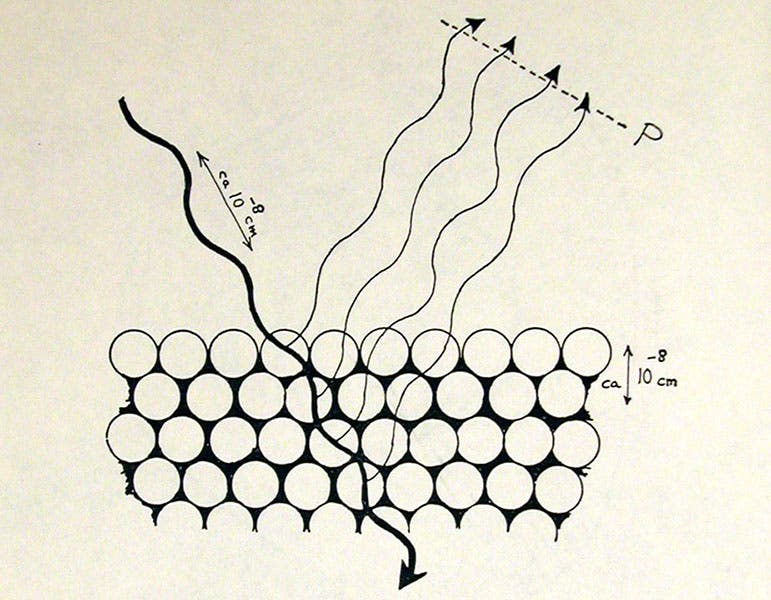
The diffraction of electron waves by a crystal lattice according to the hypothesis of Louis de Broglie, drawing by George Gamow in his Thirty Years that Shook Physics, 1966 (author’s copy)
So de Brogie proposed the existence of electron waves in his dissertation of 1924, Recherches sur la théorie des quanta (Research on the Theory of the Quanta). He even predicted the wavelengths of his proposed electron waves. It is said by several sources (which I have not been able to confirm) that the thesis examiners, unable to evaluate the proposal, sent it off to Einstein, who gave it his enthusiastic approval, and de Broglie was awarded his doctorate. It was still just a hypothesis, but fortunately, it was a testable one. The wave nature of light is easily confirmed by diffracting it with a grating, producing interference patterns, which only occur with waves. One might be able to make electron waves interfere as well. But to produce interference patterns with electron waves, if they exist, would be more difficult, because the predicted wavelengths were very short, too short to be affected by any grating that humans can produce. Fortunately, it had already been shown that X-rays, with short wavelengths, can be diffracted by crystals – nature's gratings – indeed, Louis had worked on this with brother Maurice. Could one bounce electrons off a fine crystal lattice and produce interference patterns, indicating that electrons have wave properties?
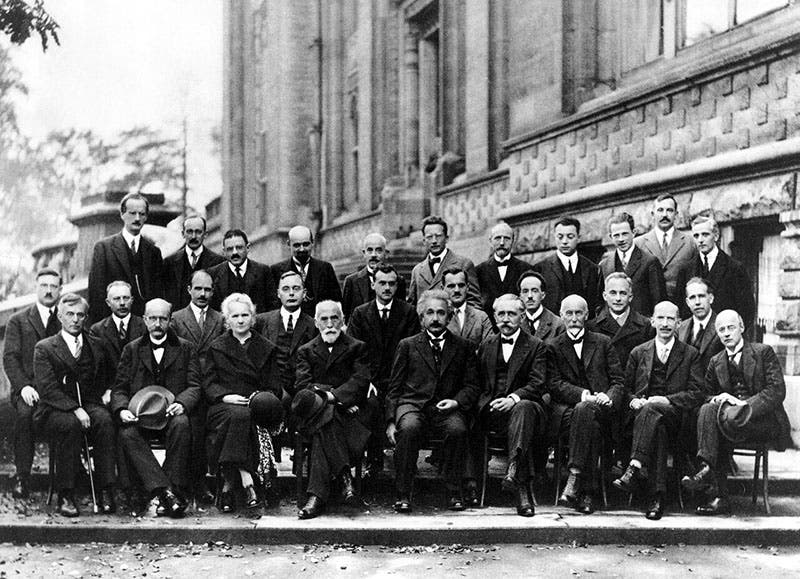
Two American physicists, Clinton Davisson and Lester Germer, did just such an experiment in 1927, using nickel crystals as a target, and they were able to detect interference patterns, and measure a wavelength for their electron waves that was just what de Brogle had predicted (third image). We wrote a post on Germer in 2022 (a similar experiment was done independently in England by George P. Thomson). The Davisson-Germer experiment would become almost as famous as the Michelson-Morely experiment of 1887 or the Millikan oil-drop experiment of 1910. It certainly brought attention to de Broglie, who was invited to the 5th Solvay Conference in Brussels in 1927, on quantum mechanics, where you can spot him in the group photo, in the second row, third from the right (fourth image). Two years later, in 1929, de Broglie was awarded the Nobel Prize in Physics for his prediction of electron waves.

The back of the dust jacket of Thirty Years that Shook Physics, with George Gamow’s sketches of the major participants in the quantum revolution, including Louis de Broglie (four o’clock position), 1966 (author’s copy)
George Gamow has an entire chapter on de Broglie in his delightful book, Thirty Years that Shook Physics: The Story of Quantum Theory, which can (and should) be read by any layman interested in the quantum revolution, in which Gamow was an active participant. Gamow related that he visited de Broglie in Paris in the late 1920s and, since de Broglie spoke no English and Gamow little French, they communicated with equations on paper. Several years later, de Broglie gave a lecture in London, which he delivered in perfect English. Gamow realized then that de Broglie had a rule: visitors to France should speak French, and visitors to England should speak English. Gamow was an inveterate sketcher, and his book provides us with own drawings of electron waves in orbit, according to de Broglie (second image), and the diffraction of electron waves by a crystal lattice (third image). The back of the dust jacket lists the major players in the story, accompanied by Gamow’s own portrait sketches (fifth image).
We have a score of books by de Broglie in our collections, written later in his career. But we do not have his thesis of 1924, one of the very few doctoral dissertations to lead directly to a Nobel Prize.
William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.