Scientist of the Day - Linus Pauling
Linus Pauling, an American chemist, was born Feb. 28, 1901. Pauling has been called, by historians who ought to know, the greatest chemist of the 20th century. His principal achievement, made in his (and the century's) late twenties, was applying the new quantum physics to structural chemistry. By the 1920s, chemists knew quite a bit about chemical bonds. They knew that many molecules are held together by bonds known as ionic bonds, the kind of bond that joins a sodium atom and a chlorine atom to form a molecule of salt. In an ionic bond, one atom essentially donates an electron to the other, and then the oppositely charged atoms are held together by electrical attraction. The other known bond, called a covalent bond, was a bit trickier. This is the bond that joins carbon atoms to atoms of oxygen or hydrogen, and in a covalent bond, the two atoms mutually share an electron or two. Predicting the structure that results from a covalent bond proved impossible. Chemists knew, for example, that in a molecule of methane (CH4), the carbon atom is at the center of a tetrahedron of hydrogen atoms, and they knew the exact angle between each of the hydrogen atoms, but they were unable to predict or explain why this was so.
At the same time, across the quad in the physics department, in universities throughout Europe, a revolution was going on, with the introduction of quantum mechanics. Physicists such as Werner Heisenberg and Erwin Schrödinger were demonstrating in the mid-1920s that the atom is quantized; that electrons can have orbits of only certain sizes and shapes, and with only certain directions of spin. Moreover, they found that a hydrogen atom could be represented by an equation – a wave equation – that predicted the probability that an electron could be found at a particular location in the atom at a particular time. It seemed possible that wave mechanics might have something to say about how covalent bonds form. But quantum physicists spoke a language entirely foreign to chemists, who still envisioned their electrons as tiny spheres with an electrical charge. The only chemist in the 1920s who tried to understand wave mechanics and apply it to structural chemistry was Pauling.
He came to Europe, studied with the leading quantum physicists, spent some time at Niels Bohr's Institute in Copenhagen, and then attacked the problem of covalent bonds. Using quantum mechanics, he found that he could predict and explain the tetrahedral structure of the methane atom and the angle between the bonds. In 1931, he published a paper called “The Nature of the Chemical Bond, application of results obtained from the quantum mechanics…,” in the Journal of the American Chemical Society. We show here the first page of that paper (second image, above), and a page with a diagram of the four electron waves, called orbitals, of the carbon atom (third image, below). This was one of the most profoundly influential papers of 20th-century chemistry, in which Pauling set down six rules, derived from quantum physics, that determined the nature of covalent bonds. The paper was followed by subsequent papers by Pauling, and in 1939, he unified these papers into a book, The Nature of the Chemical Bond (1939), which was to chemistry what Maxwell's A Treatise on Electricity and Magnetism (1873) had been to electrodynamics – a revolutionary work that re-established the field for a new generation. It was for his work on chemical bonds that Pauling received the Nobel Prize in Chemistry in 1954. Surprisingly, we do not have the first edition of his milestone book in our collections, although we have all the individual papers.
In the 1930s, Pauling turned to molecular biology – indeed, he helped found the discipline, studying the molecules of life and trying to figure out their structure and how they work. One of his coups in this field was unraveling the nature of a protein structure, known as the alpha helix. A protein is typically a long molecule, folded up into a secondary structure that is maintained by weak hydrogen bonds. Pauling was able to predict the structure of the alpha helix, publishing his solution in 1951. Pauling was at the time ensconced at Caltech in Pasadena, and he and his team were in a race with the Cavendish Lab at Cambridge in England, headed up by Lawrence Bragg. Pauling’s success was quite a coup for the Pasadena group, and a crushing defeat for the Cavendish men and women.
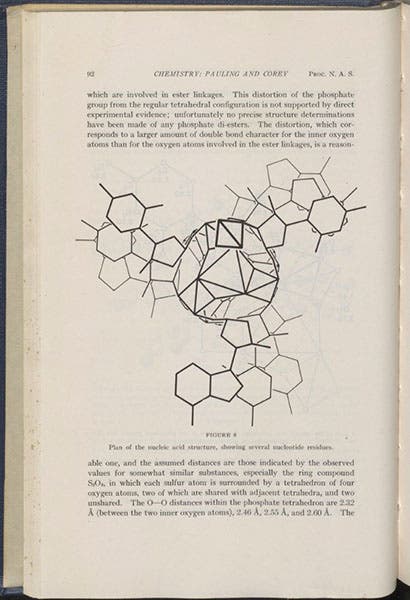
One year later, in late 1952, Pauling decided to jump into the pool of molecular biologists trying to determine the structure of DNA. He knew that the Cavendish Lab was working on the same task, and he wanted to beat them again to the finish. And he thought he had done so. Early in 1953, he published a paper, with Robert Corey, in which he announced that the DNA molecule is a triple helix, with three long strands in the center, with the bases sticking out. I had never looked at this paper, so, for this occasion, I pulled out volume 39 (1953) of the Proceedings of the National Academy of Sciences, and photographed the first page (fourth image, above), as well as a diagram showing the proposed triple helix of DNA in section, with the bases on the outside (fifth image, below). James Watson received a preprint and cried out in glee that the master had got it wrong. And indeed he had. The correct solution, a double helix with the bases inside, was announced just a few months later, in the classic paper by Watson and Francis Crick.
Pauling was forgiven for this gaffe – his Noble prize came just a year later – but his career had taken a dramatic turn. He became very active in efforts at nuclear disarmament, which was certainly commendable, and which resulted in the award of the Nobel Peace Prize in 1962, a singular achievement (or should we say a double achievement) that is still unprecedented. But then, in the 1970s, he began a crusade promoting megadose vitamin-C therapy for curing cancer. It is difficult to understand his fervor, since there was no evidence to support such claims, and as he touted his miracle cure on talk shows and late-night TV, it was hard to realize that this wild huckster had been the most brilliant mind in chemistry in the first half of the century. It has been claimed that losing the competition to discover the structure of DNA changed Pauling, and sent him off on roads that were best left untraveled. Perhaps. Or perhaps he just got old – he would not have been the first brilliant scientist to misplace his common sense as he moved into his 70s and 80s.
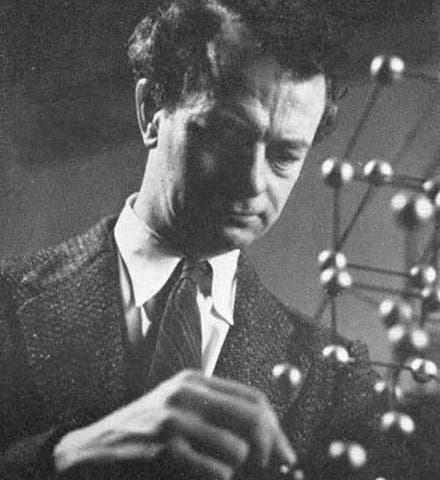
But I prefer not to dwell on Pauling’s later career. Nothing can take away the genius of his first 53 years of life. I prefer to remember Pauling as he was in the 1940s – sharp as a tack, and looking it, as our second portrait photo demonstrates (sixth image, just above)
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.







![Using an astrolabe to measure the depth of a well, woodcut in Elucidatio fabricae vsusq[ue] astrolabii, by Johannes Stöffler, 1513 (Linda Hall Library)](https://assets-us-01.kc-usercontent.com:443/9dd25524-761a-000d-d79f-86a5086d4774/a998eb50-55d2-4a88-ace2-a50aa5fa86e7/Stoffler%201.jpg?w=210&h=210&auto=format&fit=crop)

