Scientist of the Day - Johann August Arfwedson
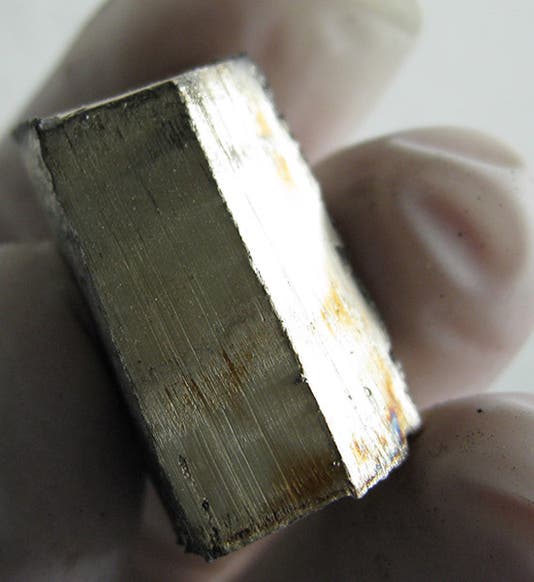
Johann August Arfwedson, a Swedish chemist, died Oct. 28, 1841, at the age of 49. He was born Jan. 12, 1792, in Skaraborg County in Sweden. Arfwedson (sometimes spelled Arfvedson) studied law and mineralogy at Uppsala, where Linnaeus had taught for so many years, and as a young graduate, he met the accomplished Jons Jakob Berzelius in Stockholm, where Berzelius maintained his chemistry laboratory. I am not sure whether Arfwedson was hired by Berzelius, or more likely, was allowed to work there, but within a year, in 1817, Arfwedson discovered a new element. He was examining a recently discovered mineral found in a mine at Utö in Sweden, and named petalite, and it seemed to contain silica, alumina, and some unknown alkali compound, a sulfate. Two new alkali metals, sodium and potassium, had recently been discovered by Humphry Davy in England; these were light metals that, in their pure form, react very strongly with water, yet formed the stable compounds of soda and potash (and table salt). Davy had discovered these using electrolysis, breaking apart soda and potash with the new battery invented by Alessandro Volta.
Arfwedson was unable to break apart his new sulfate, but he was able to show, by a variety of precipitation tests, that the new metal was neither sodium, potassium, nor magnesium, so it must be something new. He and Berzelius decided to call it lithion (lithium), because it was discovered in a mineral (lithic means rocky), whereas sodium and potassium were first isolated from animal and vegetable compounds.
Berzelius announced the discovery first, in the Journal für Chemie und Physik (vol. 21, 1817), in a short article titled (in German) "A new mineral alkali and a new metal,” in which he gave full credit to Arfwedson (fourth image) and described him as a "young, very meritorious chemist, who has worked in my laboratory for a year," and where he also recounted how they arrived at the name lithium for the yet-to-be isolated pure metal. Arfwedson published his own, much longer paper in the Annales de chimie et de physique (vol. 10, 1819), in a paper titled (in French) "Analysis of some minerals from the mine of Utö in Sweden, in which has been found a new fixed alkali." Since we have both these papers in our serials collection, and since we have little else by way of graphics for today, I show the beginnings of both papers here, as well as a detail from Berzelius’s article where he praises young Arfwedson (third to fifth images).
Arfwedson was not able to isolate pure lithium, because he didn't have enough battery power to electrolytically break apart his alkali sulfate. That was accomplished by the English chemist, William Thomas Brande, in 1821, who attacked lithium oxide with a bank of batteries powerful enough to do the job.
Scandinavians discovered lots of new elements from local ores in the 19th century, mostly rare earths like yttrium, which are very similar in their occurrence and behavior and really make no difference to anyone except the semi-conductor industry. We tend not to showcase those discoveries here, since they lack general interest, important though they were in filling out the yet-to-be-ordered periodic table. But lithium is different. This is the third element on the periodic table, right after hydrogen and helium, the two lightest but most important elements in the universe, the principal components of stars. Some lithium atoms are probably primordial, meaning they date back to the Big Bang and the origins of our universe. Lithium is more commonly a byproduct of fusion in stars; if you smash two helium atoms together and spit out a proton, you have lithium. But most of the lithium produced that way is then recombined to make carbon and oxygen and loses its lithic identity. Origin-of-the-elements experts think that most of the lithium on Earth today consists of fragments from more massive elements, chipped off by cosmic rays. It is not especially rare, which is a good thing, given the growing trend in lithium-battery-powered electric vehicles.
Arfwedson doesn’t seem to have done much more in chemistry. He inherited an estate and spent most of his time managing that. We have only the one portrait, a lithograph, which we show as our second image.
William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.