Scientist of the Day - James Chadwick
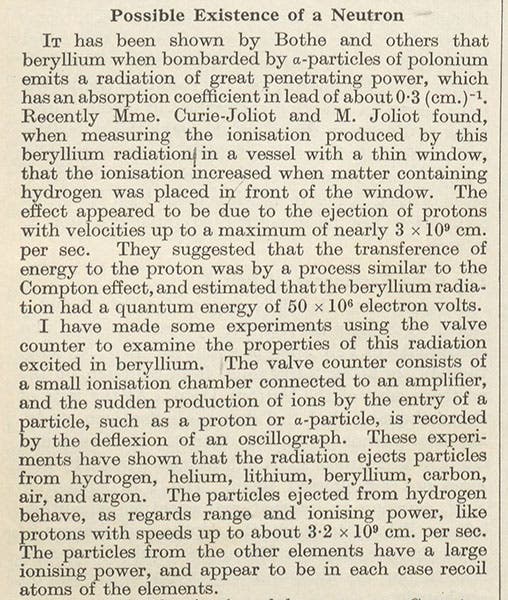
James Chadwick, an English physicist, was born Oct. 20, 1891. In 1920, Chadwick's mentor, Ernest Rutherford, proposed the existence of a neutral nuclear particle, a combination of a proton (a particle that Rutherford had discovered) and an electron. For eleven years, Chadwick toyed with experiments that might reveal such a particle, without success. Then, late in 1931, he learned that Irène and Frédéric Joliot-Curie in Paris had bombarded a light element, beryllium, with alpha particles from a polonium source, and had produced some energetic radiation. They thought, and so announced, that this radiation was gamma radiation, similar to that produced by the newly discovered cosmic rays. When Chadwick saw the Joliot-Curie publications, he knew that there was something wrong with their interpretation – the energies were not right. Having just acquired some polonium of his own, he redid the Joliot-Curie experiments, this time sending the radiation into chambers containing various light elements, and he measured how much they rebounded from the radiation. He discovered that the energy they picked up was considerable and could best be accounted for by a nuclear particle with about the mass of a proton, but no charge. In February 1932, he published a short paper, "Possible Existence of a Neutron" in the journal Nature (second and third images). The long-sought neutron had been discovered, and named. The physicist Emilio Segrè said of Chadwick: "To his great credit, when the neutron was not present, he did not detect it, and when it ultimately was there, he perceived it immediately, clearly and convincingly. These are the marks of a great experimental physicist."
Interestingly, the name “neutron” had already been coined for another purpose, by Wolfgang Pauli, who in 1930 predicted the existence of a mass-less, charge-less particle. But since his predicted particle had not yet been discovered, the name had not yet been used, and so Chadwick snatched it for his neutral particle. When Pauli’s “neutron” was eventually discovered (in 1956), it was called instead a “neutrino”, or “the little neutral particle,” really a more appropriate term for this wee bit of nothing.
Chadwick’s discovery of the neutron was a notable instance of a major discovery being made by one individual, with much of the work being done by others who misinterpreted the results. But the Joliot-Curies did not begrudge Chadwick his Nobel Prize in Physics, which he received in 1935. For they received their own Nobel Prize in the very same year (in Chemistry), for their discovery of artificial radioactivity. One of the Nobel photos of 1935 (fourth image, above) shows Chadwick, on the left, with a typically grim expression, next to the Joliot-Curies in the center. Most of the posed photos of Chadwick show him as stiff and ill-at-ease; our opening photo is an exception. Another is a remarkable photograph taken in Germany just after Chadwick published his neutron paper, which included George de Hevesy, Hans Geiger, Lise Meitner, Ernest Rutherford, and Otto Hahn (fifth image, below). Six years later, Hahn would split the atom, and Meitner would explain what Hahn had done. That must have been quite a garden party!
In the early 1940s, Chadwick was actively involved in the race to build an atomic weapon, serving as a British envoy to the Manhattan Project, and he spent a great deal of time both at Los Alamos and in Washington, D.C. When the Trinity plutonium “gadget” was detonated on July 16, 1945, the initiator at the heart of the bomb, which supplied neutrons to get the chain reaction started, was a tiny plutonium-beryllium device nearly identical to the one that Chadwick used in 1932 to discover the neutron in the first place. Chadwick was there at Trinity on that fateful day. I am sure the irony of the situation did not escape him.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.