Scientist of the Day - Ernest T. S. Walton
Ernest Thomas Sinton Walton, an Irish physicist, was born Oct. 6, 1903. Walton attended a college in Belfast, studied physics at Trinity College, Dublin, and did his graduate work at Trinity College, Cambridge, working with Ernest Rutherford at the Cavendish Lab. He received his PhD in 1931 and stayed on as a fellow at Cambridge, working on an experiment, now famous, with John Cockcroft, which produced dramatic results on this day, Apr. 14, in 1932.
At that time, it was known, primarily through the work of Rutherford, that atoms have a very tiny nucleus that contains nearly all the mass of the atom. Physicists knew about alpha particles, which are given off in radioactive decay, and which behave very much like helium atoms without their electrons. They also knew (thanks to another Rutherford experiment of 1919), that if you bombard nitrogen atoms with alpha particles, you can produce oxygen atoms, effectively transmuting the atom, the old alchemical dream. This same experiment produced the first free positively charged particles, which Rutherford called "protons," and which appeared to be constituents of the atomic nucleus, and of the alpha particle as well.
In the Rutherford experiment of 1919, Nature provided through radioactivity the fast-moving alpha particles that knocked protons from nitrogen atoms, but Nature didn't much care when that happened and what direction they went. Rutherford wanted to find a way to control the production of fast-moving particles and aim them at targets, in order to further probe the nature of the atomic nucleus. He gave Cockcroft and Walton the assignment of figuring out how to do this. Cockcroft and Walton wondered if it were possible to accelerate protons to such a high speed that when they struck an atom, they could eject particles from the nucleus and effect a transmutation this way, completely artificially. To do so, they would need a proton accelerator, and there were no particle accelerators in 1930, so they had to design and build one.
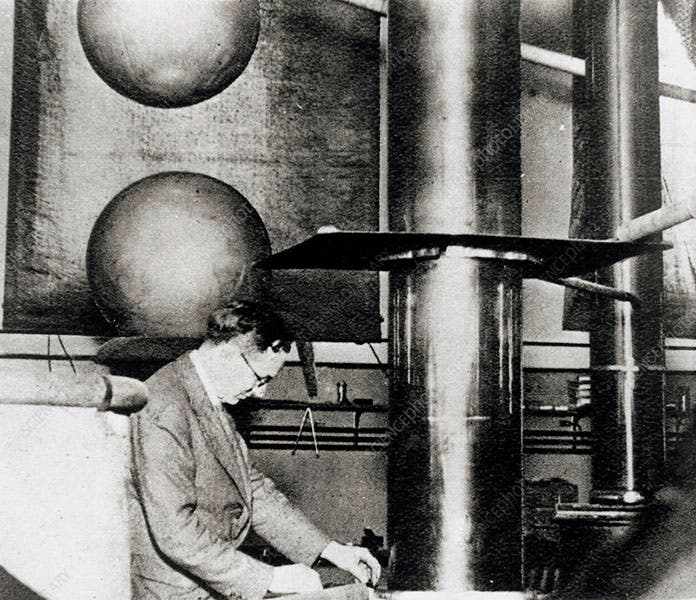
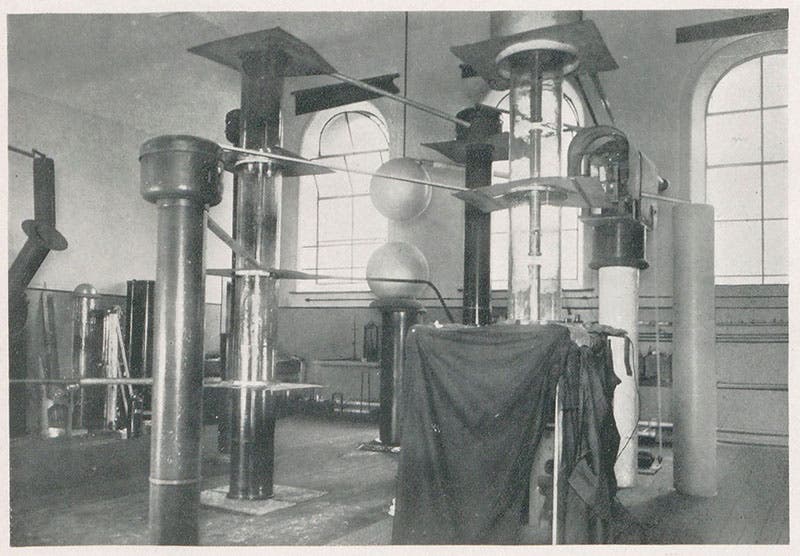
After several concepts for an accelerator did not pan out, the two physicists decided to build what is now called a voltage multiplier. One way to accelerate a proton is to put it in a tube and apply a large voltage to the tube, so that the positively charged proton will be attracted to the negative pole at the other end. If it gets going fast enough, it can be directed to a target and interact in some way. But to penetrate the positive barrier of the atomic nucleus, it will need a great deal of energy, perhaps 500,000 electron-volts. This is not easy to obtain, especially on a shoe-string budget, which all Cavendish experimenters had to live with. The Cockcroft-Walton design was brilliant and simple, at least in theory (third image). They began with a generator producing alternating current. Utilizing an ingenious arrangement of rectifiers and capacitors, arranged in stages, they could multiply the volage across the capacitors, again and again. As the voltage grew, the current dropped, but they didn't need much current, just a few microamps. The problem was insulation – a half-million volts will break down a lot of barriers, so the capacitors, which they built from scratch, were huge and isolated, and so were the rectifiers, which transformed the alternating current into direct current. Eventually, they filled an entire room at the Cavendish with their equipment. At the center was the vertical accelerator tube, across which the voltage was applied. The protons were fed in at the top, accelerated down the tube, and directed to a target at the bottom. Surrounding the target was a small box, protected from outside light, where Walton could crouch and look at a scintillation screen. If any alpha particles were produced, little dots of light would twinkle on the screen where the alpha particles impacted.

Concluding paragraph of letter by John Cockcroft and Ernest Walton, "Disintegration of lithium by swift protons," Nature, vol. 129, Apr. 30, 1932, where they concluded that accelerated protons struck and were absorbed by lithium atoms, causing each of them to split into two alpha particles (Linda Hall Library)
Fixing all the glitches took years, and that was Walton's job – he was the genius at tinkering, sealing all the vacuum leaks and redesigning the rectifiers when high-voltage sparks cracked the glass. But on April 14, 1932, he inserted a target of lithium atoms in the path of the proton beam, crawled into the box, and saw the scintillation screen aglow in sparkles, indicating a bath of alpha particles. Lithium has an atomic number of 3 and an atomic weight of 7. When a proton penetrated a lithium nucleus, producing an atomic weight of 8, the unstable nucleus broke up into two alpha particles, each with an atomic weight of 4. The atom had been split by an artificially produced beam of protons. Walton ran to alert Rutherford and Cockcroft, and everyone agreed on what was happening. Within two days, Cockcroft and Walton fired off a letter to Nature, with the title: "Disintegration of lithium by swift protons." Dated Apr. 16, it was in print by the end of the month. As you can see in a detail from the last paragraph of their short letter (fourth image), they knew exactly what they had done – they had split the lithium atom into two alpha particles.
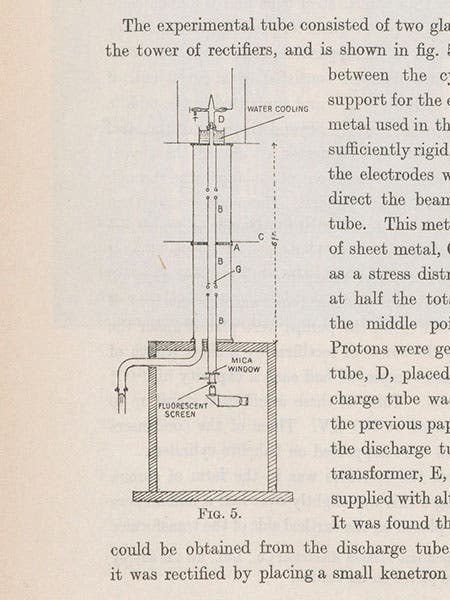
Two more-detailed papers, one on the generator and one on the results with the lithium target, were published in the Proceedings of the Royal Society of London that spring. We reproduce a diagram from the first paper that showed the observation box, the target, and the scintillation screen (fifth image).
Many Cockcroft-Walton generators were built for the major physics labs in the 1930s and 40s, and some are preserved in science museums, like the one in the Science Museum, London (first image). They are much showier than the cobbled-together device built by Walton at the Cavendish Lab in 1931.
Walton withdrew from the frenzied activity of Cavendish Lab in 1934 to return to Trinity College, Dublin, as a professor, which had always been his dream, even though it took him away from the cutting edge of particle physics. But he loved teaching, and was very good at it, and he remained in Dublin for the rest of his career. He was quite surprised to learn, in 1951, that he and Cockcroft had been chosen to share the Nobel Prize in Physics for developing their generator and using it in 1932 to disintegrate the lithium atom.
Walton died on June 25, 1995, at age 91. There are several blue plaques honoring Walton scattered about, but we like the one in Belfast, where he did his undergraduate studies (sixth image).
In our post on Cockcroft, which was written 7 years ago and was much briefer than this one, we showed an often-reproduced photograph of the Cockcroft-Walton generator at the Cavendish Lab, with a person occupying the observation box. We identified the individual as Cockcroft, because that is what our source said. It now appears that it was Walton in the box. He related later that he always crawled along the floor to get there, for fear that if he stood up, he would be electrocuted. You had to be plucky to be a particle physicist, back in the days.
Walton plays a major role in the story told by Brian Cathcart in his book, The Fly in the Cathedral: How a Group of Cambridge Scientists Won the International Race to Split the Atom (2004). Cathcart aimed his book at a general audience and was quite successful in retelling the events of 1932, in my opinion. The “fly” in the cathedral is the tiny nucleus within the atom.
William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.