Scientist of the Day - Ernest Rutherford
Wikipedia

Ernest Rutherford, a New Zealand physicist working in England, was born Aug. 30, 1871. Rutherford came to Cambridge, England, in 1895, just when the world of physics exploded, with first the discovery of X-rays, then radioactivity, and then the electron, and Rutherford took an active part in understanding all these new phenomena. It was Rutherford who announced in 1903 that when a uranium atom gives off alpha or beta particles, it actually transmutes into a different element, such as thorium. He also discovered that radioactivity seems to produce helium, suggesting that an alpha particle is some form of a helium atom. We discussed Rutherford’s work on radioactivity in our post about Bertram Boltwood last month.
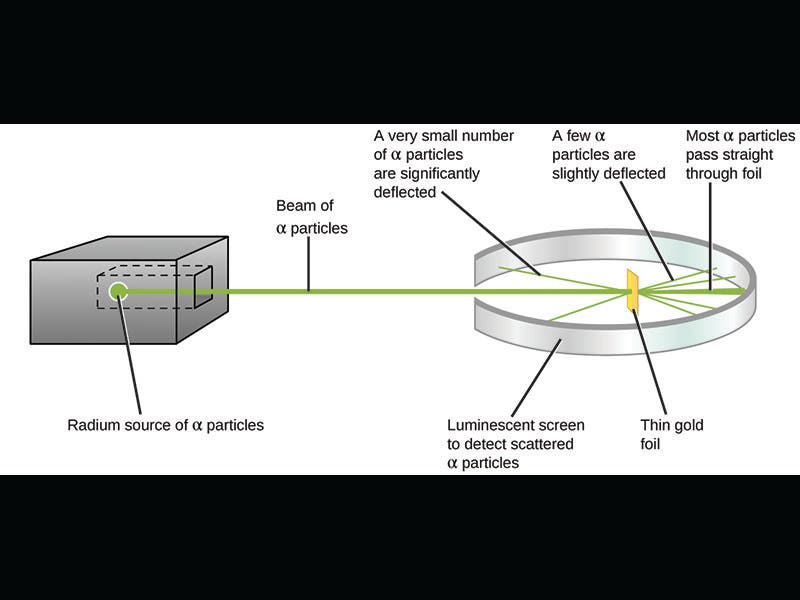
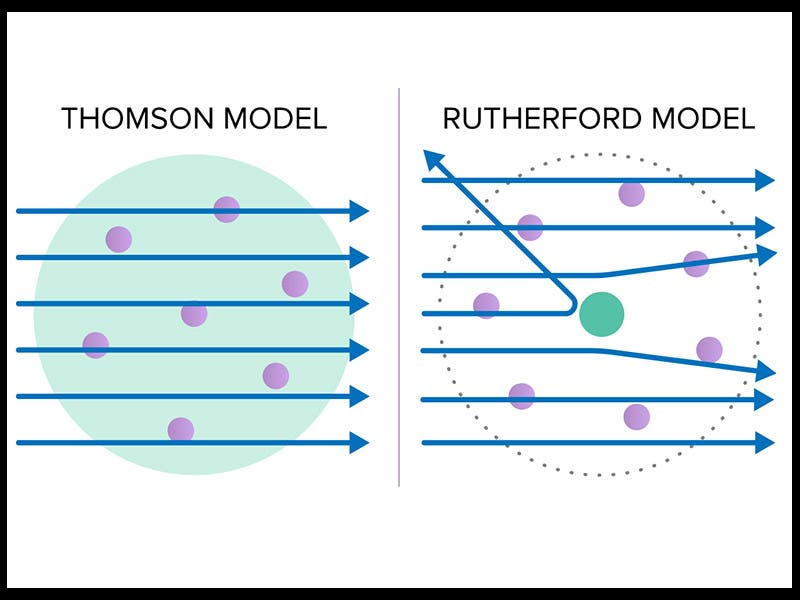
In 1911, Rutherford announced what is perhaps his greatest discovery, the atomic nucleus. Rutherford was then at Manchester, and he and his assistants designed an experiment in which a radium source emitted alpha particles that were directed to a target, a very thin sheet of gold foil. The target was surrounded by a detector that would produce a tiny luminous blip when struck by a charged particle. You can see the experimental set-up in the first diagram above. The purpose was to see whether any of the particles would be deflected by the gold atoms, which might allow them to work out the distribution of matter inside an atom. Rutherford was astonished when some of the particles were deflected at extremely large angles; it was almost as surprising as if one fired a shell at a piece of tissue paper and it bounced straight back. But Rutherford understood the implication: it must be that almost all of the mass of the atom is concentrated at the center, in a nucleus. If so, most particles would pass right through an atom, but any that chanced to strike the nucleus would bounce right back. The other diagram above (third image) shows the old atomic model at the left, called the Thomson atom or the raisin-pudding atom, with the electrons scattered throughout a positive mass, just like raisins in a pudding. Such an atom could not possibly deflect a heavy alpha particle. The Rutherford model, on the right, with its nucleus, could easily do so.
Rutherford announced the experimental evidence for an atomic nucleus in a paper in the London Edinburgh and Dublin Philosophical Magazine (or the Phil Mag, as it was called), in 1911. We have this journal in our serials collection. Rutherford probably would have won a Noble Prize for the discovery of the nucleus, but he had already received the Prize three years earlier, for his work on radioactivity.
Rutherford had made two momentous discoveries, and he was still in second gear. He would later discover the proton, and train a new generation of physicists. We will have more to say about Rutherford’s later career on a future anniversary.
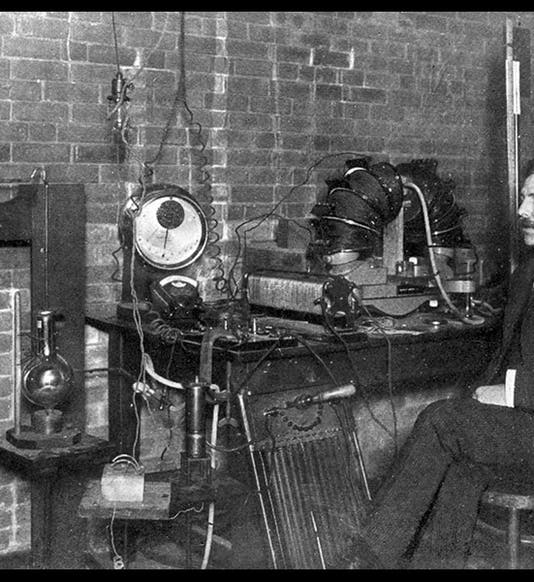
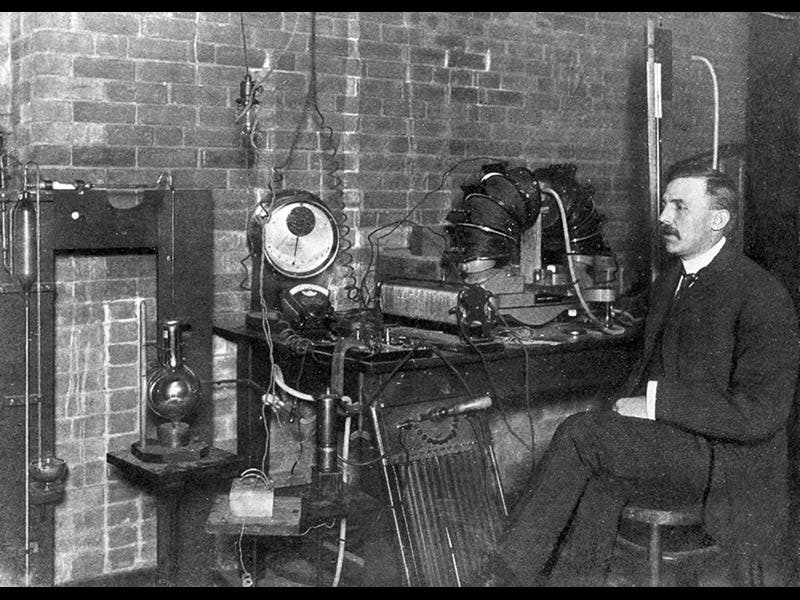
The photograph shows Rutherford at age 34, in his lab at McGill University, Canada, where he worked on radioactivity prior to moving to Manchester. The oil portrait (fourth image), painted by James Gunn in 1932, is in the National Portrait Gallery, London.
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.