Scientist of the Day - C.V. Boys
Charles Vernon Boys, an English physicist, was born Mar. 15, 1855. Boys (who always went by his initials, C.V.) was a classical physicist who could have been out of place in the age of Einstein and Bohr, but wasn't. He liked building devices and instruments, and measuring things better than anyone else could do, and he did not care that he was not contributing to cutting-edge theoretical physics. He was one of the first to use a stroboscope to capture a bullet in flight, long before Harold Edgerton perfected the technique. He invented a camera with two lenses mounted 180 degrees apart on a whirling disk, in order to photograph lightning; the two images, taken micro-seconds apart, would, he hoped, show whether lightning bolts shot up from the earth or down from the sky. Interestingly, the first time he used it successfully was when he visited the wealthy American patron of physics, Alfred Loomis, in 1930, and was given the run of his lab at Tuxedo Park, New York. In our post yesterday on Vannevar Bush, in the final photograph, showing the war-time scientific brain trust of 1940, Loomis was the person at far right.
One of Boys’ greatest inventions was the fused quartz torsion balance. A torsion balance depends on a fine thread, which will twist one way or the other when parts of the balance are subjected to forces. The virtue of a torsion balance is that it can measure extremely small forces. The finer the thread, and the less torsion it takes to twist it, the more sensitive it will be. Boys discovered that when you fuse (melt) quartz, it can be drawn out into a fine but strong thread that is perfect for torsion measurements. The difficulty is in drawing out the thread before it cools off and solidifies. Boys solved the problem by attaching a quartz rod to the bolt (arrow) of a crossbow, fusing it, and then shooting it down a long room at high speed with the crossbow. The quartz thread that was strung out in its wake was so fine that you could not even see it. He used the resulting quartz fiber to build a balance with which to replicate the century-old experiment of Henry Cavendish, who determined the gravitational constant by measuring the attraction of two small spheres. Boys’ measurement was much better than that of Cavendish, and deviated just 0.5 percent from the modern value.
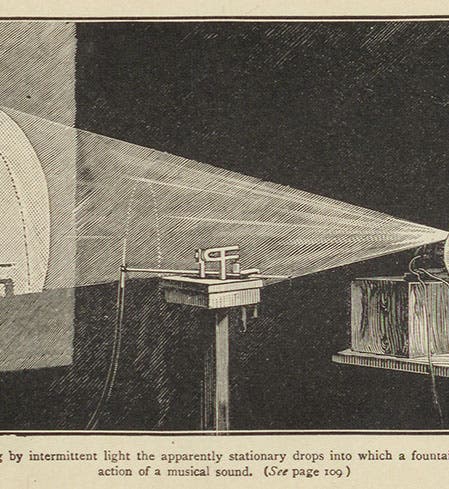
But perhaps Boys' greatest legacy was a book called: Soap Bubbles and the Forces Which Mould Them (1890), which was the product of three lectures he gave at the Royal Institution in London in 1889 and 1890. Our Library has a 1907 edition (third image). As Boys says in the introduction, the book is for young people, and anyone who is not young should go away and do something else. It is basically a book about surface tension, although I am not sure he ever uses that word, because his main intent is to show that physical science is fun, and amazing, and can be pursued by anyone who can tie a shoe. The frontispiece (first image) replicates one of his Royal Institution demonstrations, in which a stream of liquid is broken up into tiny drops by the sound of a tuning fork; the droplets become visible when illuminated by a strobe light. At the end of the book, Boys appended a long section in which he gave directions for performing every experiment that he described. One of his experiments involved building wire forms, in the shapes of cubes and tetrahedrons and other polyhedra, and then dipping the forms into a soap solution. The surface tension of the soap film will pull the bubbles into beautiful and surprising shapes (fourth image).
Did Soap Bubbles attract anyone to experimental science? Well, let me tell you. Boys' book was republished in 1959 (fifth image) as one of the first books in the new Science Study Series (which we discussed briefly in our post on Arthur Koestler not long ago). I splurged 95 cents on a copy, read it right through, and was fascinated, especially by the wire forms. The high-school science fair was coming up, so I made four different polyhedral forms out of wire. I built a crankshaft from wooden dowels that was about two feet long, hooked it up to a 4-rpm motor, attached each of the forms to one of the cranks, and suspended the shaft over a long wallpaper-paste trough filled with soap solution. When a visitor to my booth pressed a button, the shaft turned, the wire forms descended one by one into the soap solution, and each came up bearing a glistening and unexpected geometric shape fashioned of soap. It was magic, I must say. I seem to remember that I won my division with my soap-shape machine, but I am not sure of that. But it doesn’t matter. Boys played no small role in sending me off to Wesleyan University to study physics. I remain grateful to his enthusiasm for interesting young people in experimental science.

Front cover, first paperback edition of Soap Bubbles and the Forces Which Mould Them, by C.V. Boys, Garden City, N.Y., Doubleday Anchor, 1959 (author’s copy)
Boys, perhaps surprisingly, was highly regarded in Edwardian and Interwar Britain. He was knighted, made a fellow of the Royal Society of London and the other important scientific brotherhoods,and his obituary for the Society was written by the 4th Baron Rayleigh. His portrait (second image) was painted in 1915 by John Maler Collier, who also did memorable portraits of Charles Darwin, Thomas H. Huxley, and William Huggins, all of which you can see at our post on Collier.
If you have children or relations looking for a science fair project, you could do worse than give them a copy of Soap Bubbles and the Forces Which Mould Them, even though it was written 132 years ago. The book is still in print, although the glitzy cover of the Dover Publications reprint leaves a lot to be desired. The Science Study Series did it better (fifth image).
Dr. William B. Ashworth, Jr., Consultant for the History of Science, Linda Hall Library and Associate Professor emeritus, Department of History, University of Missouri-Kansas City. Comments or corrections are welcome; please direct to ashworthw@umkc.edu.